Injectable Concentrated
The combination of these two technologies allows the production of an enriched conditioned serum (Concentrated Autologous Conditioned Serum)., resulting in a substantial quantity of anti-inflammatory cytokines suitable for a single treatment. Injectable Concentrated Conditioned Serum (iCCS) is cell-free and abundant in anti-inflammatory cytokines and growth factors.
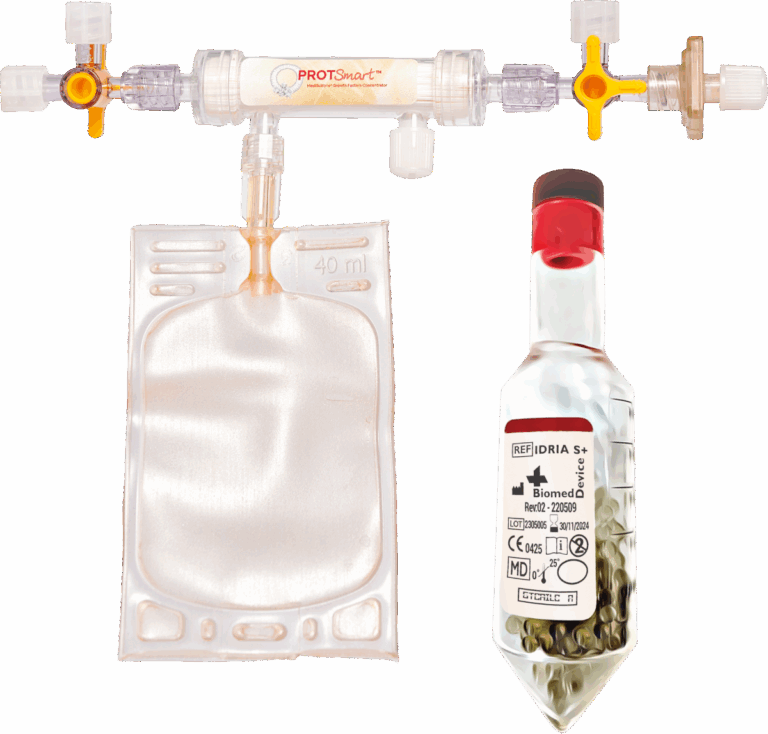
This treatment is designed to support a balanced inflammatory response, promote tissue homeostasis, and help maintain joint integrity. It may also contribute to cellular health and overall wellness associated with healthy aging. Physicians can process 30 ml of blood and wait just 30 minutes for activation, then obtain 15ml of Injectable Concentrated Conditioned Serum with high concentrations of anti-inflammatory cytokines in a single spin (2,700 rcf (×g) for 10-minute). Afterwards, physicians can further process all serum components with ProtSmart 2 to achieve 2-3 ml of hyper-concentrated conditioned serum, enriched with proteins, growth factors, IL-1Ra, IL-4, and IL-10. This process takes only a few minutes to be ready for direct injection into patients.
The Power of
An autologous cytokine serum that promotes anti-inflammatory effects and tissue regeneration. The system employs glass beads coated with positively charged ions to stimulate monocyte activation, inducing the release of anti-inflammatory cytokines.
What Sets IDRIA S+ Apart?

IDRIA S+ provides a hyper-concentration and cellular stimulation of IL-1Ra directly from the patient’s own blood, eliminating the need for any incubation process. The presence of glass beads in IDRIA S+ activates monocytes to produce anti-inflammatory cytokines such as IL-1Ra and enhances the concentration of anti-inflammatory cytokines IL-4 and IL-10. IL-1Ra, known as interleukin-1 receptor antagonist, is a naturally occurring cytokine that inhibits inflammation by binding to the IL-1 receptor, thereby preventing its activation. IL-1Ra not only blocks IL-1 but also exhibits analgesic and neuroprotective properties. It helps maintain proteinase balance and, in synergy with IL-4 and IL-10, promote homeostasis in the microenvironment and tissue (including cartilage) synthesis.
What is
ProtSmart combines advanced capillary membrane filtration with blood product concentration technology.
Its specially engineered capillary membrane, with an average pore size of 15,000 Da, efficiently removes plasma water while retaining platelets and essential growth factors. The process is simple and optimized requiring only one centrifugation step to separate and discard red blood cells. With ProtSmart, both PRP and PPP can be concentrated by reducing volume by up to 70%, while preserving the plasma proteins crucial for tissue regeneration.
ProtSmart 2
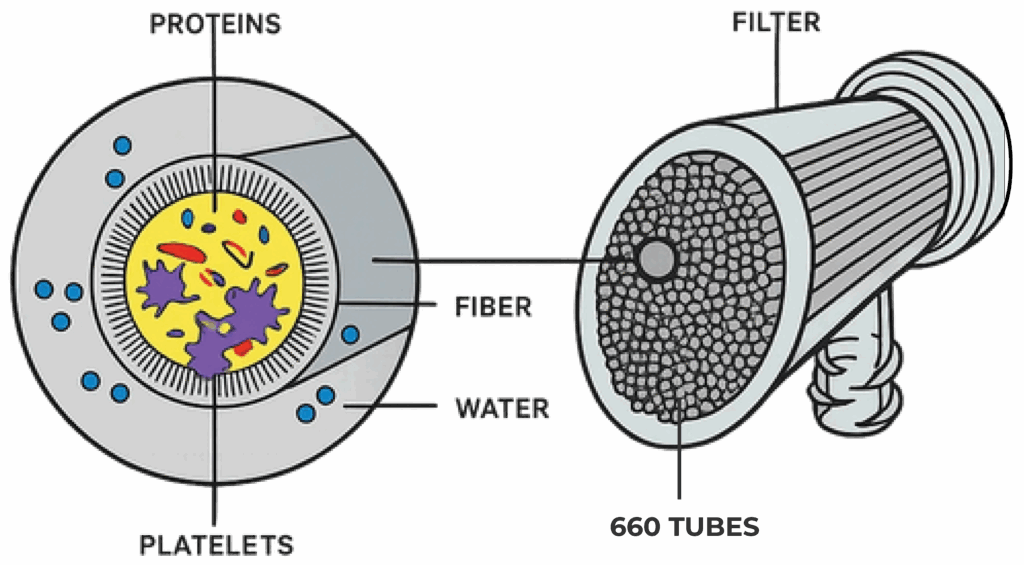
The PRP and PPP preparation protocols are different from one another, but they generally consist of the collection of autologous blood, the centrifugation of tubes or syringes to separate red blood cells from plasma and other cellular components such as leukocytes and platelets and in isolation (by other follow up centrifugations or by means of a selective aspiration) the plasma phase enriched with platelets (PRP) or poor in platelets (PPP).

Idria S+ & iCSS
Within the interleukin family, IL-1 is a pro-inflammatory cytokine released by immune cells during the early phases of inflammation. Its action can be regulated by IL-1Ra (interleukin-1 receptor antagonist), a naturally occurring cytokine released by monocytes. IL-1Ra binds to the IL-1 receptor, inhibiting the interaction between the receptor and the agonist, thereby preventing IL-1 intracellular signaling. IL-1Ra blocks the pro-inflammatory stimulus and promotes tissue regeneration.
The combination of these two technologies allows the production of an enriched conditioned serum without the need for incubation, resulting in a substantial quantity of anti-inflammatory cytokines and exosomes suitable for a single treatment.
WHY CHOOSE INJECTABLE CONCENTRATED CONDITIONED SERUM (iCCS):
KEY CLINICAL SETTINGS
Autologous cytokine preparations are being explored in fields such as dermatology, urology, and orthopedics, where clinicians may use them to support tissue homeostasis and recovery.
Total Protein Concentration Test of Protsmart 2
Reference: Medica
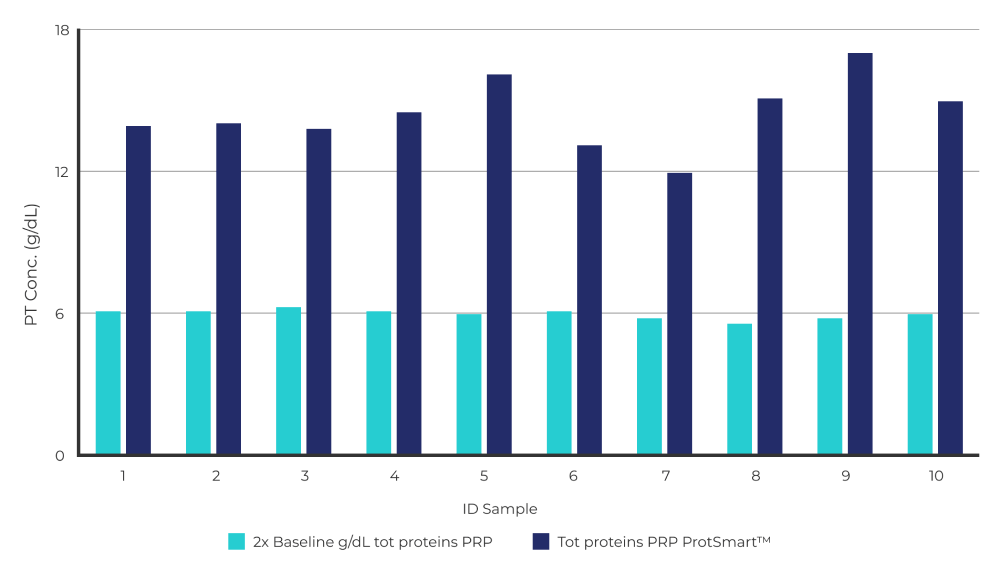
Total protein concentration in basal PRP and concentrated PRP measured by ProtSmart. The total plasma proteins including autologous growth factors increase their concentration by about 2.2-3 times compared to the basal PRP concentration obtained by a single centrifugation.
Concentrated
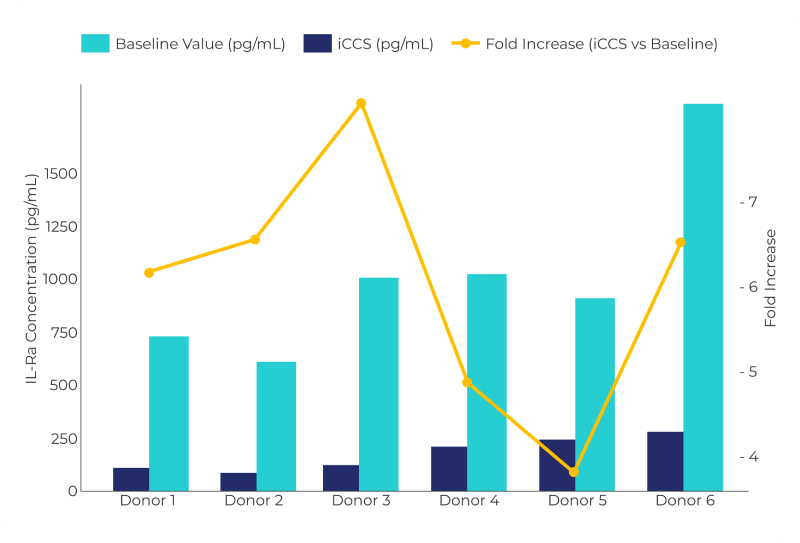
In all the experiments there is an increase of ILR1a versus the basal with an average increase of 6,4 with a standard deviation of 1,5 and CV% of 25%. The basal average value is 175 pg/ml, confirming literature data of ILR1a in the whole blood. Data show a good average increase in ILR1a content compared to baseline: the low standard deviation, that measures how much the values of a data set deviate from their media, assure of the excellent reproducibility of the data.
EXOSOME CONCENTRATION TESTS
Reference: SSCB Swiss Stem Cells Biotech AG
iCCS INDICATIONS
OSTEOARTHRITIS
These therapies are commonly used to treat osteoarthritis, a degenerative joint disease characterized by cartilage breakdown, inflammation, and pain. By reducing inflammation and modulating the immune response, these treatments can help alleviate pain and potentially slow down the degenerative process.
RHEUMATOID ARTHRITIS
Rheumatoid arthritis is an autoimmune condition causing joint inflammation, pain, and potential joint deformity. While these therapies have been less commonly used for rheumatoid arthritis, they may help manage symptoms by reducing inflammation and pain.
GYNAECOLOGY
These therapies could potentially be explored for their effects on inflammatory gynecological conditions such as endometriosis or pelvic inflammatory disease, where reducing inflammation may help alleviate symptoms.
SPORTS-RELATED INJURIES
These treatments may be used for joint, ligament, or muscle injuries sustained during sports activities, with the aim of promoting healing, reducing inflammation, and shortening recovery times.
POST-SURGICAL JOINT RECOVERY
These therapies could be used to improve joint recovery following surgical procedures, such as arthroscopy or joint replacement, by reducing inflammation and promoting tissue repair.
HAIR LOSS
While more commonly treated with platelet-rich plasma (PRP) therapy, it is possible that these therapies could be investigated for their potential role in promoting hair follicle health and regrowth.
TENDINOPATHIES
Inflammatory conditions of the tendons, such as tendonitis or tendinosis, may benefit from these therapies. They could potentially reduce inflammation and promote tissue healing.
UROLOGY
Their anti-inflammatory properties might be beneficial in the context of chronic prostatitis, interstitial cystitis, or other inflammatory urological conditions.
DERMATOLOGY
The anti-inflammatory properties of these therapies might be helpful in managing inflammatory skin conditions such as psoriasis or atopic dermatitis.
TECHNICAL SNAPSHOT*
INPUT
30 mL whole blood → IDRIA S+ → 15 mL conditioned, cell-free serum
TIME
30 min activation
SPIN
10 min at defined rcf) - No incubation
ULTRAFILTRATION (PROTSMART)
Membrane 15 kDa MWCO; reduce to a clinician-set target (e.g., 3-8 mL)
RESIDUAL CELLS (SPEC)
Low residual RBC/granulocytes in S+ serum before concentration
* Operational parameters/yields vary with technique, equipment, and donor factors. Refer to IFUs.
Quality &
- Parameters defined: time/rcf/volumes; membrane settings and target volumes
- Documentation: IFU/SOP, QC & batch templates, traceability
- Labeling: multilingual inserts/labels where applicable
IMPORTANT INFORMATION
Regulatory status: CE-marked where applicable. Availability, permitted uses, and labeling vary by country.
For professional use only. Product availability, regulatory status, and indications vary by market. Consult the IFU and local regulations before use.
Kit



- Idria S+ glass bead tube FOR CONDITIONING
- ProtSmart ultrafiltration module
- Blood-draw accessories (by configuration)
- Quick protocol card + IFUs
