
The Science Behind
Scientific Background
The regenerative capacity of the human body relies on a highly organized biological signaling system.
Cells communicate through a constant exchange of biochemical messages, enabling damaged tissues to initiate repair, regulate inflammation, and restore function.
Among these signaling mediators, exosomes — nanosized extracellular vesicles (30–150 nm) — have emerged as key regulators of intercellular communication and tissue regeneration.
Exosomes are secreted by most cell types, including platelets, which are particularly rich in regenerative mediators.
These vesicles carry messenger RNA, microRNA, and proteins that influence how recipient cells behave, controlling processes such as angiogenesis, fibroblast proliferation, collagen synthesis, and immune modulation.
Unlike traditional platelet-rich plasma (PRP), which relies primarily on soluble growth factors released after platelet activation, autologIX focuses on isolating and concentrating the exosomal fraction, where the most precise regenerative information resides.
Exosomes are not just an additive to PRP — they are the biological language through which platelets and cells orchestrate healing. autologIX is designed to extract this language from plasma — capturing the body’s own exosomes and preserving them in their natural biological environment.
By combining exosomes with their supporting plasma components (growth factors, fibrin, cytokines, and proteins), The autologIX provides a stable, potent, and reproducible autologous therapy.
Scientific Rationale
Several studies have demonstrated that exosomes derived from autologous blood components replicate and even exceed the biological effects traditionally attributed to PRP.
Their nanometric size allows them to penetrate tissue barriers, reach target cells, and regulate gene expression directly.
The regenerative process involves three key biological domains — cell signaling, matrix remodeling, and immune regulation — and exosomes act simultaneously in all three.
Signal Amplification
Exosomes function as messengers, delivering specific regulatory microRNAs and proteins that activate transcription factors in recipient cells, upregulating genes related to proliferation, angiogenesis, and collagen synthesis.
Matrix Remodeling
Exosomes stimulate fibroblasts and mesenchymal progenitor cells to rebuild organized extracellular matrix while maintaining balance between synthesis and degradation.
Immune Modulation
By influencing macrophage polarization and cytokine release, exosomes reduce pro-inflammatory signaling and promote resolution of inflammation, creating a microenvironment suitable for repair.
The autologIX replicates this physiology in a clinical setting by concentrating exosomes in a plasma fraction that retains the necessary co-factors for stability and function — a “biological microenvironment” that mirrors how exosomes act inside the body.
Preparation of
The procedure begins with a 48 mL blood draw divided into four 12 mL sterile tubes designed specifically for regenerative preparation.
Each tube contains a separation gel and anticoagulant that preserve platelet structure and ensure clear plasma separation.
After centrifugation, the upper platelet-rich plasma (PRP) layer is collected. This plasma contains a mixture of platelets, soluble proteins, and exosomes that circulate naturally in the bloodstream.
It forms the biological base for the autologIX process, which concentrates and refines this fraction into a standardized exosome-rich preparation.
Because the procedure uses only the patient’s own blood, the final product remains 100 % autologous — ensuring compatibility, safety, and stability without any additives or foreign substances.
AutologIX Advantages
8
MIN
Process Time
100%
AUTOLOGOUS
95%
SUCCESS RATE
ProtSmart 6
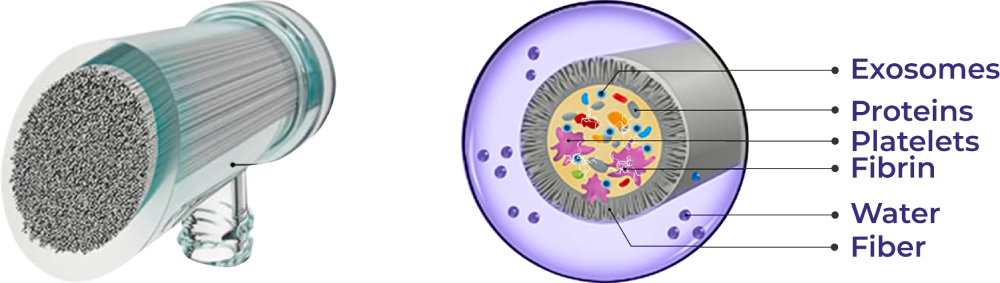
At the core of autologIX lies the ProtSmart 6 ultrafiltration device — a precision system that separates plasma components according to molecular size, selectively retaining the exosomal and protein-rich fraction.
REGENERATIVE FACTOR STRUCTURE INSIDE THE PROTSMART 6 FILTER
MULTI-TUBE STRUCTURE FOR PRECISE ACTIVATION

800 Tubes
CROSS-SECTIONAL STRUCTURE OF THE HOLLOW FIBER FILTER
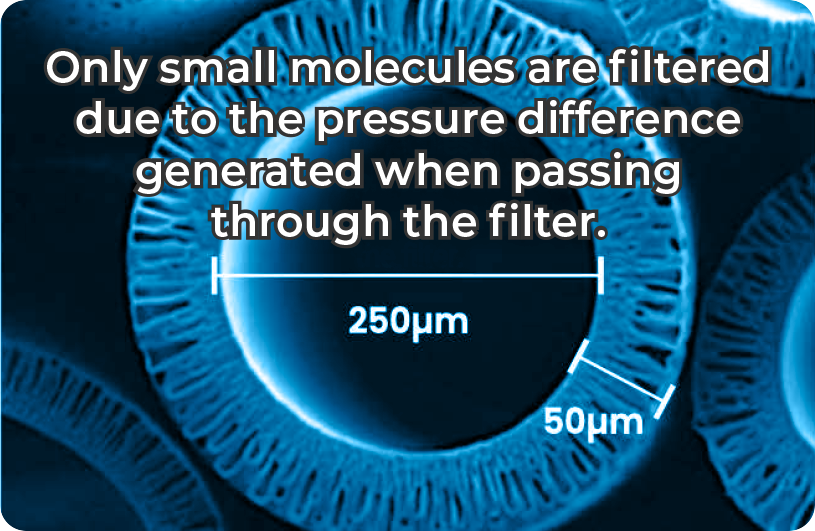
MEDISULFONE UF SEM IMAGE
(COXEM, EM30AX 300X)
ULTRAFILTRATION TECHNOLOGY OF PROTSMART 6
Use of high-precision ultrafiltration filter (≤ 5nm)
Preservation of only effective components above 15 kDa
Removal of unnecessary moisture, salts, and anticoagulants, etc.
Technical Specifications:
- Filtration cut-off: 15 kDa
- Average pore diameter: ≤ 5 nm
- Process time: ≈ 8 minutes
- Input plasma: 4 × 12 mL tubes (≈ 48 mL total)
- Output: ≈ 6–7 mL exosome-rich autologous plasma concentrate
The hollow-fiber ultrafiltration membrane allows small molecules (water, salts, and anticoagulants) to pass through while retaining larger components — exosomes, platelets, fibrin fragments, cytokines, and proteins.
A gentle pressure gradient and low-shear conditions protect the vesicles from rupture or degradation.
During centrifugation, platelets are a ctivated in a controlled manner, releasing additional exosomes and growth factors into the retained plasma.
The process yields a homogeneous concentrate with a high density of exosomes expressing surface markers CD9, CD63, and CD81, confirming their extracellular vesicle origin.
This technology bridges two critical needs in regenerative medicine — biological potency and standardization.
It enables the reproducible preparation of an autologous exosome concentrate that maintains biological integrity and consistency across treatments.
ProtSmart 6
SCIENTIFIC VALIDATION
30T/mL Exosomes
Secures approximately 30 trillion exosomes per mL from autologous blood
Nanoparticle tracking analysis (NTA)
[Reference] 1. StemExoOne Co., Ltd., Independent Validation Report, 2024
Uniform Exosome Size
Confirmed uniform size and structural consistency
Distribution of Particles
Transmission Electron Microscopy
Growth Factor
Generates up to 4.8 times more effective growth factors compared to conventional PRP
Multiplex Growth Factor Quantification (5-Plex)
- PRP
- ProtSmart 6
(Unit: pg/mL)
[Reference] 1. Wongbee MedTech Report (2025-027-L.5Plex).
ProtSmart 6 CD Marker
Confirmed expression of effective exosome markers (CD9, CD63, CD81): proven high positive rates
Flow Cytometry
[Reference] 1. StemExoOne Co., Ltd., Independent Validation Report, 2024
Mechanisms of
The biological activity of autologIX is determined by an integrated set of processes involving exosome-mediated communication, molecular signaling, and matrix remodeling.
A. Cellular Communication
Once administered, exosomes attach to or fuse with recipient cell membranes, delivering their cargo of regulatory RNAs and proteins.
These signals stimulate intracellular pathways such as PI3K/AKT, ERK/MAPK, and STAT3, promoting cell survival, proliferation, and differentiation. In fibroblasts, this results in increased synthesis of collagen I and III; in endothelial cells, it leads to neovascularization; in stem/progenitor cells, it enhances commitment to regenerative lineages.
B. Growth Factor Cooperation
Platelet-derived growth factors — PDGF, VEGF, TGF-β, IGF-1 — act synergistically with exosomes. They amplify tissue response by attracting cells to the injury site, stimulating matrix deposition, and improving vascular supply. Together, they create a coordinated biological program for regeneration, where exosomes provide the molecular instructions and growth factors provide the biochemical support.
C. Inflammation Resolution
Exosomes contained in autologIX influence macrophage polarization by suppressing pro-inflammatory cytokines (IL-1β, TNF-α) and increasing anti-inflammatory mediators (IL-10, TGF-β). This immune modulation limits tissue damage and accelerates the transition from inflammation to remodeling. Clinically, this correlates with decreased swelling, improved perfusion, and early pain relief.
D. Structural Remodeling
The combined presence of fibrin, exosomes, and growth factors forms a temporary three-dimensional scaffold where cell migration and matrix organization occur. The end result is a structurally integrated, vascularized, and functionally mature tissue rather than fibrotic scar formation
Clinical Fields
Because of their universal role in cellular signaling, exosomes extend across a wide therapeutic spectrum.
MUSCULOSKELETAL AND ORTHOPEDIC MEDICINE
Applied in tendinopathies, muscle tears, ligament injuries, and early osteoarthritis. Exosomes promote chondrocyte metabolism, reduce synovial inflammation, and accelerate tendon remodeling, improving functional recovery.
WOUND HEALING
In chronic or post-surgical wounds, exosomes stimulate fibroblast proliferation, endothelial sprouting, and keratinocyte migration. The anti-inflammatory action re-establishes balance in non-healing wounds and shortens the time to closure
DERMATOLOGY AND AESTHETIC MEDICINE
Used for facial rejuvenation, scar remodeling, and inflammatory skin conditions. Exosomes reactivate dermal fibroblasts, restoring collagen and elastin synthesis and improving skin tone, texture, and elasticity.
HAIR RESTORATION
In androgenetic alopecia, exosomes enhance dermal papilla cell activity, extend the anagen phase, and improve scalp vascularity. Repeated sessions support visible hair density and thickness improvements.
PAIN AND INFLAMMATORY DISORDERS
By acting directly on inflammatory mediators and promoting genuine tissue repair, autologIX provides a biological alternative to pharmacologic pain control in osteoarthritis, tendinopathies, and chronic soft-tissue injuries.
Clinical Advantages
Targets the key messengers that drive regeneration.
Derived exclusively from the patient’s plasma; No additives or donor materials.
Retains all components > 15 kDa, ensuring dense exosome and protein yield.
ProtSmart 6 guarantees process uniformity and consistent composition.
Approximately 8 minutes from plasma to final concentrate.
Simple venipuncture and local administration; no downtime.
Applicable across orthopedic, dermatologic, wound-healing, and aesthetic medicine.
No immunogenic or allergic reactions due to full autologous origin.
